Ice Cream – The Science: When salt is added to ice, it lowers the freezing point of the ice, making it colder than normal ice. This allows it to absorb more heat from the ice cream mixture, helping it freeze faster. When you shake the bag, the heat from the warmer ice cream is absorbed by the colder ice, which helps the blend cool down and freeze into solid ice cream. Shaking the bag also keeps the mixture in motion, preventing the formation of large ice crystals and resulting in smooth, creamy ice cream rather than a solid block of ice.
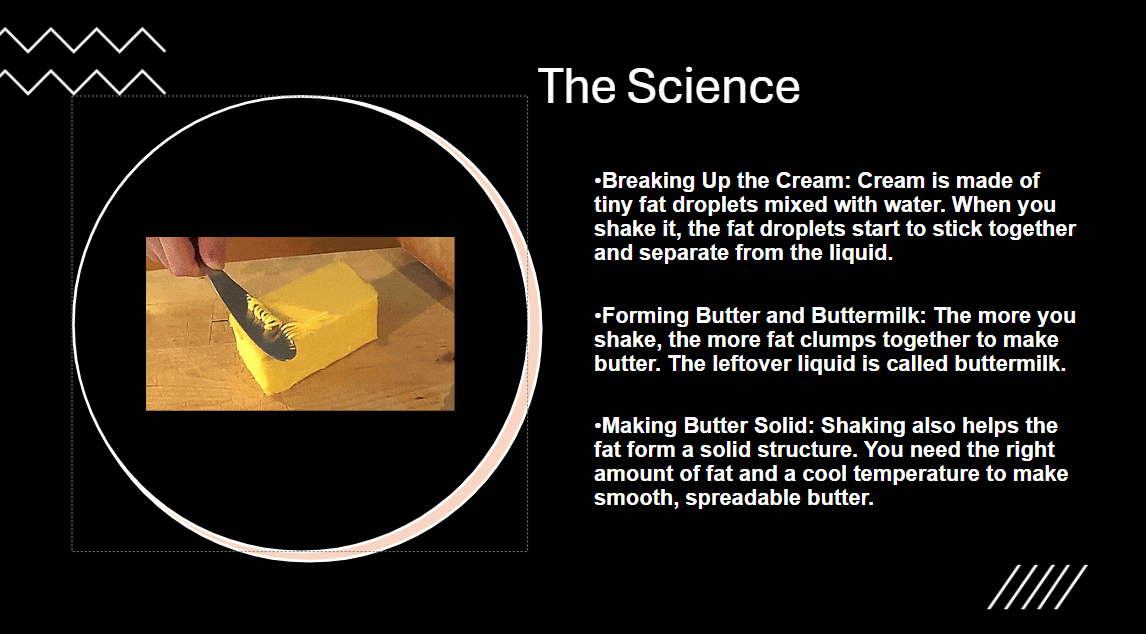
Butter – The Science: Cream is made of tiny fat droplets mixed with water. When you shake it, the fat droplets start to stick together and separate from the liquid. The more you shake, the more fat clumps together to make butter and the leftover liquid is called buttermilk. Shaking also helps the fat form a solid structure. You need the right amount of fat and a cool temperature to make smooth, spreadable butter.